The Importance of Accuracy and Precision in Chemistry
Accuracy and precision are two critical concepts in the field of chemistry that play a significant role in ensuring the reliability and validity of experimental results.
Accuracy
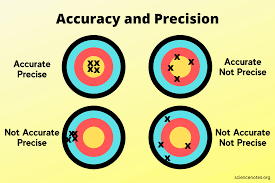
Accuracy refers to how close a measured value is to the true value or accepted standard. In chemistry, accurate measurements are essential for obtaining meaningful data that can be used to draw valid conclusions. A high degree of accuracy minimises errors and uncertainties in experimental results, leading to reliable findings.
Precision
Precision, on the other hand, relates to the consistency or reproducibility of measurements. A precise measurement is one where repeated trials yield similar results. In chemistry, precision is crucial for assessing the reliability of data and determining the level of confidence in experimental outcomes.
Relationship Between Accuracy and Precision
While accuracy and precision are distinct concepts, they are interrelated. Ideally, measurements should be both accurate and precise to ensure the credibility of experimental findings. However, it is possible to have measurements that are precise but not accurate (due to systematic errors) or accurate but not precise (due to random errors).
Significance in Chemistry
In chemistry, accuracy and precision are vital for various purposes, such as determining the composition of substances, measuring reaction rates, analysing environmental samples, and developing new materials. Reliable data obtained through accurate and precise measurements form the foundation for scientific discoveries and advancements in the field.
Conclusion
In conclusion, accuracy and precision are fundamental principles that underpin the practice of chemistry. By striving for both accuracy and precision in experimental work, chemists can enhance the quality of their research outcomes, foster scientific progress, and contribute to a deeper understanding of the natural world.
Essential FAQs on Accuracy and Precision in Chemistry
- What is the difference between accuracy and precision in chemistry?
- Why are accuracy and precision important in chemistry?
- How can accuracy be improved in chemical measurements?
- What factors can affect the precision of experimental results in chemistry?
- Can a measurement be precise but not accurate in a chemical experiment?
- What techniques are used to assess the accuracy of analytical instruments in chemistry?
- How do systematic errors impact the accuracy of chemical measurements?
- What role does uncertainty play in determining the precision of data obtained from chemical experiments?
- Why is it necessary to calibrate equipment regularly to maintain both accuracy and precision in chemical analyses?
What is the difference between accuracy and precision in chemistry?
In the realm of chemistry, a common query revolves around distinguishing between accuracy and precision. Accuracy pertains to how close a measured value aligns with the true or accepted standard, ensuring the reliability of experimental data. On the other hand, precision refers to the consistency or reproducibility of measurements, highlighting the degree of agreement among repeated trials. Understanding this disparity is crucial in conducting experiments that yield dependable results and draw valid conclusions in the intricate world of chemistry.
Why are accuracy and precision important in chemistry?
Accuracy and precision are crucial in chemistry for ensuring the validity and reliability of experimental results. Accuracy is essential as it determines how close a measured value is to the true value or accepted standard, allowing scientists to draw meaningful conclusions based on accurate data. Precision, on the other hand, measures the consistency and reproducibility of measurements, indicating the reliability of experimental outcomes. In chemistry, the combination of accuracy and precision is vital for producing trustworthy data that forms the basis for scientific discoveries, advancements, and innovations in various fields of study.
How can accuracy be improved in chemical measurements?
Improving accuracy in chemical measurements involves several key strategies. Firstly, calibrating instruments regularly and using standard reference materials can help ensure that measurements align closely with accepted values. Additionally, employing proper techniques, such as careful sample handling and accurate data recording, can minimise errors and enhance the accuracy of results. Conducting multiple trials and averaging the outcomes can also improve accuracy by reducing the impact of random errors. Furthermore, verifying the accuracy of results through comparison with known standards or other analytical methods can provide validation and confidence in the measured values. By implementing these practices diligently, chemists can enhance the accuracy of their chemical measurements and produce reliable data for scientific analysis and interpretation.
What factors can affect the precision of experimental results in chemistry?
In the realm of chemistry, several factors can influence the precision of experimental results. One significant factor is the quality and calibration of measuring instruments used in the experiment. The accuracy and reliability of tools such as pipettes, burettes, and balances directly impact the precision of measurements obtained. Additionally, variations in experimental conditions, such as temperature fluctuations, environmental factors, and human error during data collection or analysis, can introduce uncertainties that affect the precision of results. Proper technique, meticulous attention to detail, and consistent methodology are essential to minimise these factors and enhance the precision of experimental outcomes in chemistry.
Can a measurement be precise but not accurate in a chemical experiment?
In the realm of chemistry, the question of whether a measurement can be precise but not accurate is a common query that highlights the nuanced nature of experimental data. In a chemical experiment, it is indeed possible for a measurement to exhibit precision without accuracy. This scenario may occur when repeated measurements yield consistent results (precision) but deviate from the true value or accepted standard (inaccuracy). Such discrepancies could stem from systematic errors inherent in the experimental setup or methodology, leading to a situation where the data is precise in terms of reproducibility but lacks accuracy in reflecting the actual quantity being measured. Understanding this distinction between precision and accuracy is crucial in critically evaluating experimental outcomes and ensuring the reliability of scientific conclusions in chemistry.
What techniques are used to assess the accuracy of analytical instruments in chemistry?
In the field of chemistry, assessing the accuracy of analytical instruments is crucial to ensure reliable and trustworthy results. Various techniques are employed to evaluate the accuracy of these instruments, such as calibration with known standards, comparison with reference methods or certified materials, proficiency testing, and internal quality control measures. Calibration involves adjusting the instrument using standard samples of known composition to establish a correlation between the instrument’s readings and the true values. Comparison with reference methods or certified materials allows for validation of results by confirming their agreement with established standards. Proficiency testing involves participating in inter-laboratory comparison exercises to assess the instrument’s performance relative to other laboratories. Internal quality control measures, such as running replicate samples and monitoring instrument drift over time, help maintain accuracy and identify any deviations that may affect results. By employing these techniques, chemists can ensure the accuracy of analytical instruments and enhance the reliability of their experimental data in chemistry.
How do systematic errors impact the accuracy of chemical measurements?
Systematic errors have a significant impact on the accuracy of chemical measurements by introducing consistent biases that cause measured values to deviate from the true value. These errors stem from flaws in experimental design, equipment calibration, or procedural techniques, leading to a systematic deviation in results. In chemistry, systematic errors can result in inaccuracies that persist across multiple measurements, compromising the reliability and validity of data. Identifying and correcting for systematic errors is crucial to improve the accuracy of chemical measurements and ensure the integrity of scientific findings.
What role does uncertainty play in determining the precision of data obtained from chemical experiments?
In the realm of chemistry, uncertainty plays a crucial role in determining the precision of data obtained from chemical experiments. Uncertainty reflects the potential variability or margin of error associated with measurements, highlighting the limitations and reliability of experimental results. By quantifying and accounting for uncertainties in data analysis, chemists can assess the level of precision in their measurements and make informed decisions about the validity and significance of their findings. Understanding and managing uncertainty is essential for ensuring the accuracy and trustworthiness of scientific conclusions drawn from chemical experiments.
Why is it necessary to calibrate equipment regularly to maintain both accuracy and precision in chemical analyses?
Regular calibration of equipment is essential to maintain both accuracy and precision in chemical analyses for several reasons. Calibration ensures that the measuring instruments are correctly aligned with known standards, allowing for accurate interpretation of experimental results. By calibrating equipment regularly, any deviations or drift in measurements can be detected and corrected promptly, thus enhancing the accuracy of data. Additionally, calibration helps to improve the precision of measurements by reducing variability and ensuring consistent performance over time. Ultimately, regular calibration of equipment is crucial in ensuring the reliability and validity of chemical analyses, enabling researchers to obtain trustworthy results that form the basis for scientific conclusions and advancements in the field.

Woah! I’m really loving the template/theme of this site.
It’s simple, yet effective. A lot of times it’s very difficult to get that “perfect balance” between usability and
visual appeal. I must say you have done a superb job with this.
Also, the blog loads extremely fast for me on Internet explorer.
Excellent Blog!
Thank you for your positive feedback! We’re glad to hear that you’re enjoying the content and design of our blogarticle on the importance of accuracy and precision in chemistry. We strive to provide valuable information in a user-friendly format. Your appreciation motivates us to continue delivering quality content. If you have any questions or topics you’d like us to cover in future articles, feel free to let us know. Thank you for reading!